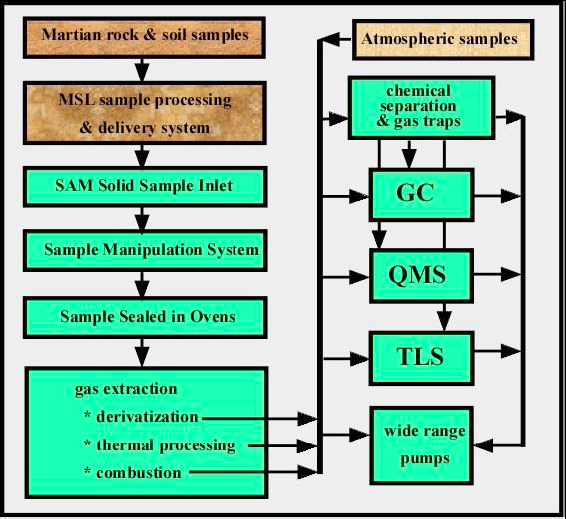
SAM – Sample Analysis at Mars
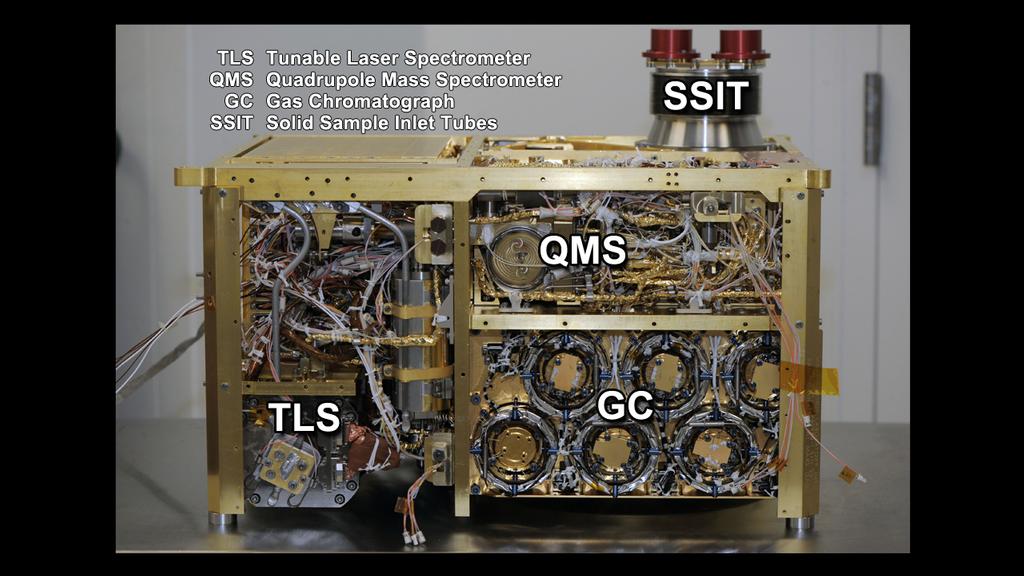
SAM is a compact instrument that facilitates a suite of sensitive chemical analyzers focused on determining elemental and molecular chemistry that is relevant to life. It will address the present and past habitability of the planet. Carbon Chemistry is provided by searching for organic compounds, the chemical state of light elements other than carbon, and isotopic tracers of planetary change.
With a mass of about 40kg, SAM is one of the heavier instruments aboard the MSL Rover. It is located inside the Rover’s body.
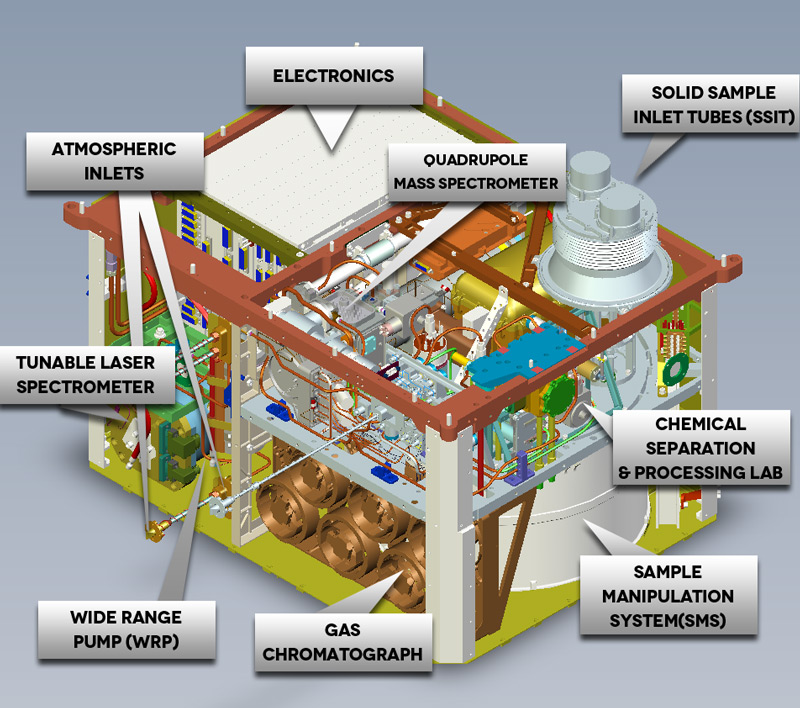
SAM includes three individual instruments: a Quadrupole Mass Spectrometer (QMS), a Gas Chromatograph (GC) and a Tunable Laser Spectrometer (TLS). QMS and GC can be operated together in a GCMS (Gas Chromatograph Mass Spectrometer) mode for separation and definitive identification of organic constituents.
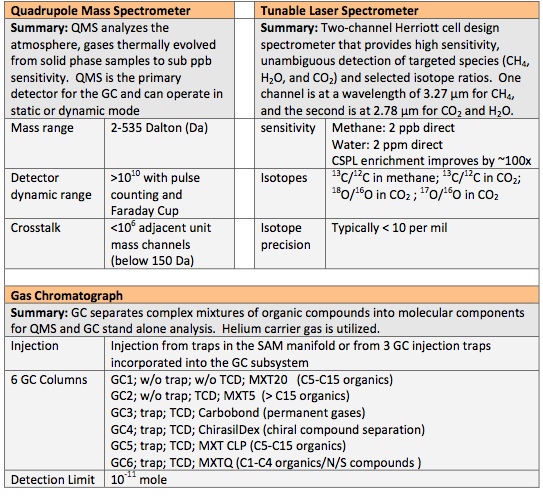
GC separates complex mixtures of organic compounds into molecular components for stand alone processing or QMS processing. For that, the Gas Chromatograph has six complementary chromatographic columns. It examines gases that are separated from mixtures of gases by pushing the mixture through long, narrow tubes with a stream of Helium gas, forcing the molecules to group by molecular weight. The lightest molecules emerge from the tubes first and are followed by heavier molecules. Once gases are sorted, they can be directed to QMS and TLS for further analysis.
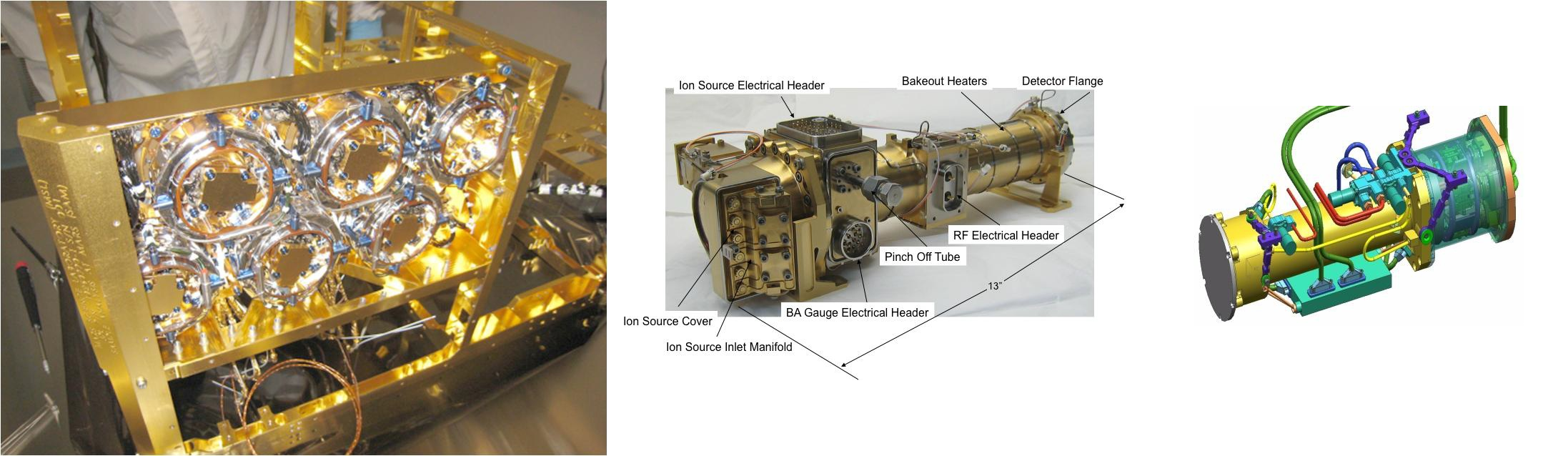
QMS analyses the atmosphere and gases that are thermally evolved from solid materials. It identifies gases by molecular weight and electrical charge of ionized particles. Electrons are fired at the molecules breaking them into fragments and sorting those by establishing an electric field. QMS provides spectra that are used to identify the molecules. QMS can operate in static or dynamic mode.
TLS uses absorption of light at specific wavelengths to provide exact isotope ratios for Carbon and Oxygen in Carbon Dioxide and it measures trace levels of methane and its carbon isotope. It is based around a two channel Herriott cell design spectrometer. The instrument has a high sensitivity of its targeted species and selected isotope ratios of 2ppb for methane and 2ppm for water.
SAM Instrument Details
SAM – Sample Cups

The SAM instruments are supported by a number of subsystems. A sample Chemical Separation and Processing Laboratory (CSPL) will sample the atmosphere. Valve and pump manipulations direct an appropriate amount of gas through an inlet tube to the SAM instrument package. CSPL includes high conductance and micro valves, gas manifolds with heaters and temperature monitors, chemical and mechanical pumps, carrier gas reservoirs and regulators, pressure monitors, pyrolysis ovens, and chemical scrubbers and getters.
The Sample Manipulation System (SMS) will transfer finely sieved samples into one of 74 sample cups that can be inserted into a SAM oven to thermally process the material releasing any gases that are the focus of analyses. The cups are stored in a wheel within the system that is capable of rotating to receive samples for a particular cup and deliver cups to the oven. For that, the wheel has to rotate with an accuracy of less than one millimeter. 59 cups on the wheel are Quartz cups that can receive samples. 9 cups contain chemical solvents for lower-temperature wet-chemistry experiments and the remaining 6 cups contain calibration materials. The solvents are used for special analyses of material rich in organics.
In addition to that, SAM carries a several calibration gases including a mix of N2, CO2, Ar, and a Xe in its 129 isotope form to distinguish it from Martian and terrestrial Xenon.

SAM Science Objectives
- Survey Carbon Compound sources and evaluate their possible mechanisms of formation and destruction. [Met by measurements of the identity and abundance of organic molecules and their state of oxidation, molecular weights and structures.]
- Search for organic constituents of biotic and pre-biotic significance, including methane. [Met by measurements of amino acids, nucleobases, carboxylic acids by chemical derivatization and solvent extraction. TLS measures methane abundance its C13/C12 isotope ratio.]
- Reveal the chemical and isotopic state of elements that are important for life. [Met by measurements of inorganic gases including Water vapor, Carbon Dioxide and Sulfur Dioxide.]
-

Photo: NASA Determine atmospheric composition including trace species that are evidence of interactions between atmosphere and soil [Met by measurements of abundances of minor and trace constituents of the atmosphere including those with short photochemical atmospheric lifetimes. Diurnal and seasonal variations of the following substances are measured: Water Vapor, Oxygen, Nitrogen, Argon, Ozone, Hydrogen and Methane.]
- Better constrain models of atmospheric and climatic evolution through measurements of noble gas and light element isotopes. [Met by measurements of atmospheric gases and gases evolved from fines and powders, in particular the isotope ratios for noble gases, Carbon (13-12), Nitrogen (15-14), Oxygen (18-16 and 17-16) and Deuterium/Hydrogen.]
Addressing these science goals will provide information of past, present and potential future habitability properties of Mars.
Science Sequences
Solid Samples
- Pyrolysis with GCMS: Quartz Cell inside Pyrolysis Oven, Sample delivered to cooled cup then heated up to 1000°C in helium gas stream, evolved gas is monitored by QMS and TLS; GCMS analysis with gas trapped during gas processing
- Derivatization: Analysis of chemically derivatized polar compounds (amino acids or carboxylic acids)
- Combustion: Analysis of carbon isotopes in CO2 produced by combustion in oxygen (sample combusted using oxygen gas, CO2 analysis by TLS for isotopic composition)
Atmospheric Analyses
- Direct Atmospheric Measurement: Atmospheric Gas directed into instrument package, analysis by QMS and TLS to obtain atmospheric chemical and isotopic composition as well as seasonal variations in trace species abundance
- Atmospheric Enrichment: Gas directed into gas traps for enrichment, QMS, TLS and GCMS analysis of atmospheric trace species
- Methane Enrichment: Atmospheric Gas directed through scrubbers and cold traps, Methane released into TLS for isotopic and abundance studies
- Noble gas Enrichment: Noble Gas Analysis with SAM instruments after gas scrubbing
Calibration Sequences
- In-Situ Gas Calibration: Calibration Gas released from storage tank, QMS ans TLS scans, fluorocarbons trapped and analyzed by GCMS, Will reveal instrument changes over time
- Solid Sample Calibration: Sample Cup containing control sample opened and delivered to oven, heated up to 850°C in helium gas stream while evolved gases are analyzed by QMS and TLS, GCMS analysis of trapped fluorocarbons (6 control sample cups are available for landed operations)
